Abstract
Introduction
Following the launch of the TKI's (tyrosine kinase inhibitors) for the treatment of CML (Chronic myeloid leukemia), establishing its significant control over the disease as evident by multiple studies such as the population based Swedish CML registry reporting that Patients reaching 70 years of age had a relative survival close to 1.0 compared to the normal population with the same age. Consequently, other dimensions have emerged regarding the safety of treatment, particularly the effect on Male fatherhood. This study was conducted to review the real-life data on the effect of TKI on the fatherhood of male patients in the National Center of cancer care and research (NCCCR) in Qatar in the period of 1st of January 2005 - 1st of January 2020. Up to our knowledge, this is the first study addressing the effect of TKI on fatherhood in patients with CML.
Methods
A single-center study, conducted a mixed-design (retrospective+ phone interviews) with CML male patients in the Chronic or accelerated phase, being followed up in NCCCR, evaluating the effect of Imatinib, Dasatinib, nilotinib, on their fatherhood whether they are taking it as first, a second, or third line of treatment.
Inclusion Criteria:
-Male patient diagnosed with CML, in Chronic or accelerated phase; 18 years of age or
older and actively receiving tyrosine kinase inhibitors including (Imatinib, dasatinib,
nilotinib) with the following:
-Patients with no known issues with regards to fertility, (fertility is intact) will be included in
the study.
-Patients who developed fertility issues after the diagnosis of CML and starting TKI's.
He has been evaluated by an andrologist and his evaluation concluded its TKI related.
Exclusion criteria:
-Patients with other MPNS.
-Patients not fulfilling inclusion criteria as follow:
-Patient known to have infertility before the diagnosis of CML
-Patient with infertility after Diagnosis of CML:
If a clear underlying cause, not TKI related, will be excluded from the study
if no evaluation was done for infertility and it is not clear whether the
infertility is related to an underlying cause or TKI and no proper evaluation by
andrologist done excluded from this study
The mother has documentation by gynecologist for infertility, or after
examining the abortion, still-birth, or IUFD and checking the chromosomal
analysis (any mother related cause whether endogenous or exogenous has been excluded)
Results:
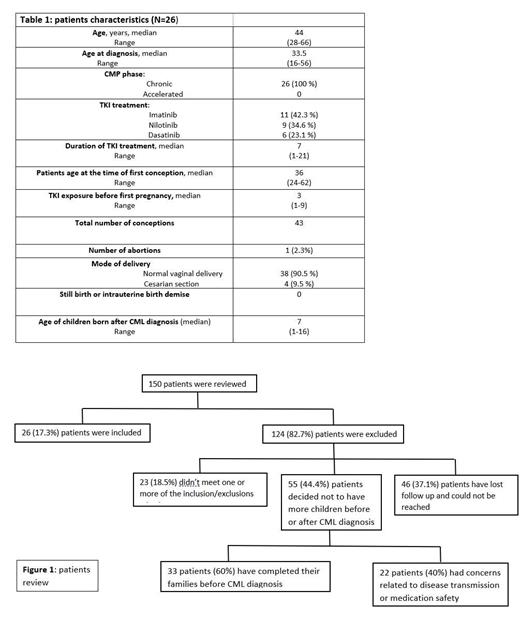
150 patients were interviewed to be included in the study, 22 (14%) patients had concerns related to medications safety and possible transmission of the disease, 33 (22%) patients had their families completed by the time of diagnosis. 26 patients have met the inclusion criteria, median age around 44 years, median age at diagnosis was 33.5. 100% were in chronic phase, 42.3% were on imatinib, 34.6% on Nilotinib, and 23.1% on Dasatinib. The median TKI exposure period before pregnancy was 3 years. Median age at first conception post TKI treatment is 36, with a median duration of TKI treatment around 7 years. offspring's total number was 43, 97.6% were full-term, had a normal delivery, and normal average weight at delivery. No stillbirths, fetal demise, or congenital anomaly were reported. All offspring had normal development and growth. Median age of children after CML diagnosis around 7 years. No reports of any CML-related cancer in all the offspring
Conclusion:
Around 98% of male CML patients taking imatinib, Dasatinib, Nilotinib had their offspring born normally with no delivery complications noted, all had no congenital anomaly, had normal growth and development, and no CML-related cancers were diagnosed. Further studies with a larger sample size are required to shed light on the TKI outcome on fatherhood in CML patients. Nonetheless, a call for attention for better education to patients starting on TKI's addressing the possible psychological fear or concerns of having an unsatisfactory effect on their fertility/offspring, targeting better acceptance and adherence to treatment.
No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal